Chronic Obstructive Pulmonary Disorder (COPD) - KOL Insight and Consensus Outlook

The cosy carve up of the market between Pfizer’s Spiriva (tiotropium) and GSK’s Advair/Seretide (fluticasone propionate/salmeterol), which currently account for 75% of sales globally, will be challenged by new entrants that provide better patient adherence, improve lung function, tackle inflammation and reduce exacerbations of the disease.
Why is COPD causing such excitement?
There is considerable industry and investor excitement about COPD treatment developments. Which of the new products will find favour with clinicians? What regulatory hurdles must they overcome? Do they offer a real clinical improvement over current therapies? Stripping away the hype and harnessing the real world insights from leading US and European KOL’s, this Therapy Trends analysis critically examines the products and research and exposes the real potential. For example:
- In September 2013, Novartis’ Ultibro (glycopyrronium/indacaterol) was the first once daily fixed-dose LABA/LAMA to be approved, with promising research results and positive KOL opinion. However, approved dosages of the indacterol element may be lower in the US, which could affect efficacy and take up. Regulatory delay with the FDA is likely to hand the advantage to GSK, Boehringer Ingelheim and Almirall/Forest all of whom are likely to bring LABA/LAMA combinations to the US market before Novartis.
- In May 2013, GlaxoSmithKline’s Breo/Relvar (fluticasone furoate/vilanterol), the first once-daily ICS/LABA was approved, but there are questions over whether it offers clinical value compared with Symbicort and Advair/Serodtide. However, the tide could turn if the product could become the first COPD therapy to gain a mortality claim if the outcome of the SUMMIT trial is positive. In the words of one KOL: “Let's assume that this study is positive. Then the whole field would change dramatically. This would cause an avalanche.”
- Takeda’s Daxas/Daliresp (roflumilast) was the first, and remains the only, oral PDE-IV inhibitor available but sales are hampered by poor tolerability, unpleasant side effects, variable US formulary coverage and restrictions on use in some EU markets. Chiesi’s CHF 6001 (Phase II) inhaled PDE-IV inhibitor is showing promising and is “extremely well tolerated in extended safety studies.”
For instant access to an accurate, unbiased, qualitative review of the latest treatment trends along with a five-year quantitative COPD market forecast look no further than FirstWord’s Therapy Trends: COPD. This comprehensive FirstWord research is available in two comprehensive modules:
- The KOL Insight: COPD module provides a complete review and is enhanced with exclusive in-depth interviews with leading KOLs from the US and Europe
- The Consensus Outlook: COPD data analysis module provides annual historical and forecast product-level sales data from an average of leading equity analysts’ projections. Key findings are provided in report which brings together data in charts and tables.
KOL Insight Report
- A qualitative analysis of the current and predicted treatment trends, clinical products and commercial changes in the global COPD market informed by in-depth expert views of leading clinicians
- Compare the market’s key players, products, late-stage pipeline drugs, product positioning, in the context of COPD market trends.
- Benefit from the latest KOL views about significant event driven changes in COPD treatment trends.
Consensus Report
- An in-depth 5-year Consensus Outlook report of predicted quantitative changes in key COPD market forecast parameters drawn from 13 leading analyst and brokerage companies
- Chart COPD market size, product sales, market share by company, competitive status and detailed market forecasts
- Use the interactive Excel market analytics module of detailed market and product sales data from 2007 to 2017 to drill down and assess key company, market and product trends
- Understand and assess future COPD market development
- Analyse current and future treatment algorithm
- Understand the market dynamics between the LAMA's, LABA's, combination ICS/LABA's and fixed dose LAMA/LABA's
- Assess how prescribing trends will change with launch of new products
- Learn about late-stage pipeline products
- Track KOL views for the next 12 months
- Evaluate future sales forecasts and predicted market growth
- Map your market parameters and chart commercial prospects
- Assess market share by company and product
- Tailor your strategic and investment focus based on the competition
- Set proactive launch and branding strategies
- Keep up with event-driven market data updates
- Business development
- New product planning
- Strategic brand planning
- Market research
- Competitive Intelligence
- Forecasting and market analytics
- Medical affairs
- Clinical trials
- Relationship management
- Market Access
- Marketing
- Sales
Chronic Obstructive Pulmonary Disease Class Dynamics
Historically market growth has been driven by Long Acting Muscarinic Antagonists (LAMAs) and inhaled corticosteroid/ Long Acting Beta Agonists (ICS/LABAs). Boehringer Ingelheim and Pfizer’s Spiriva (tiotropium) accounted for 98 percent of the worldwide LAMA market in 2012, while GlaxoSmithKline’s Seretide/Advair (fluticasone propionate/salmeterol) represented 71 percent of the ICS/LABA market share.
The other main class in COPD was the Short Acting Beta 2 Agonist/Short Acting Anticholinergic (SAMA/SABA), which is comprised of Combivent (salbutamol/ipratropium; Boehringer Ingelheim) and used in conjunction with the long-term maintenance LAMA or ICS/LABAs.
Chronic Obstructive Pulmonary Disease Class Key Class Worldwide Market Sales Value, 2012 And 2017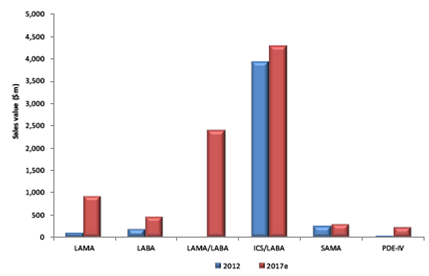
Seebri (glycopyrronium; Novartis) forecast
Novartis’s Seebri (glycopyrronium) is the latest Long Acting Muscarinic Antagonist (LAMA) to be approved in Europe. US approval is expected in 2014. Novartis licensed the formulation of glycopyrronium from Vectura/Sosei. Glycopyrronium is administered in the Breezhaler DPI, the same device used to deliver Novartis’s Arcapta/Onbrez (indacaterol). Clinical trials have showed Seebri provides rapid bronchodilation following the first dose. In 2012, Seebri generated $61 million in the European markets where it is approved.
Sales dynamics of Seebri (glycopyrronium; Novartis), 2012-2017,_2012-2017.png)
Symbicort (budesonide/formoterol; AstraZeneca) forecast
Symbicort (budesonide/formoterol; AstraZeneca) became the second inhaled corticosteroid/Long Acting Beta Agonist (ICS/LABA) combination behind Seretide/Advair (fluticasone propionate/salmeterol; GlaxoSmithKline) to be approved in the respiratory markets. An approval in chronic obstructive pulmonary disease (COPD) followed in the EU in 2003 and in the US in February 2009.
Sales dynamics of Symbicort (budesonide/formoterol; AstraZeneca), 2007-2017,_2007-2017.png)

