Products, Technologies and Markets Worldwide for the Clinical Management of Obesity, 2011-2019
13 Dec 2010 • by Natalie Aster

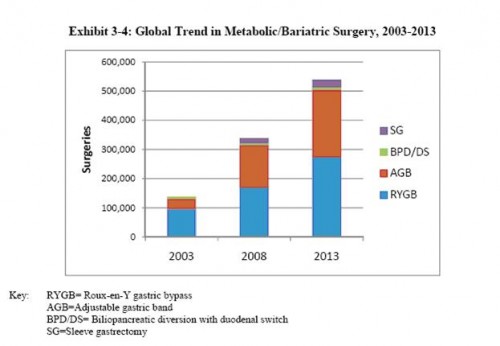
“The total number of bariatric surgery procedures is forecast to reach about 539,299 by 2013, growing by about 16.3%. Adjustable Gastric Banding, despite slowing, is expected to have the greatest compound annual growth rate for 2004-2013, at about 23%. This will be followed by the growth rate for gastric bypass at 12.4%. Biliopancreatic diversion is forecast to be nearly flat at 1.4% growth through the same time period.”
The report “ Products, Technologies and Markets Worldwide for the Clinical Management of Obesity, 2011-2019 ” by MedMarket Diligence, LLC is a detailed market and technology assessment and forecast of the products and technologies in the clinical management of obesity (bariatrics). The report details the products on the market and the status of those in development for bariatric surgery, drug therapy, gastric stimulation devices, brain stimulation devices, combination therapies and genetic therapy and other therapies under development, and will provide current and worldwide market forecasts (2009-2019) separately for pharmaceuticals and devices, with current market shares of the leading competitors in each segment.
Report Details:
Products, Technologies and Markets Worldwide for the Clinical Management of Obesity, 2011-2019
Published: December 2010
Pages: 302
Price: USD 3,750
Recent Developments Impacting Market Forecasts:
The market for obesity treatments has been on a roller coaster during 2010. Several recent incidents directly and dramatically affected market forecasts. These included:
- January - Sibutramine was pulled from the market in the EU due to concerns over an increased risk of cardiovascular events.
- June - Novo Nordisk announced the decision to re-initiate Victoza’s (liraglutide) global Phase 3 development program for the treatment of obesity. The program and clinical testing are expected to recommence in the first half of 2011.
- July - Vivus’ Qnexa is not recommended for approval by the FDA’s advisory committee due to concerns about a lack of safety data longer than one year, and concerns about birth defects if women taking Qnexa become pregnant.
- October - In the face of FDA concerns about increased risk of cardiovascular events, Abbott withdraws sibutramine from the market in the USA.
- The FDA upholds the advisory committee’s vote on Qnexa and rejects the drug, requesting more data from Vivus.
- Another FDA advisory committee votes to reject Arena Pharmaceuticals’ lorcaserin.
- November—China, India, New Zealand withdraw sibutramine from their markets.
More information can be found in the report “ Products, Technologies and Markets Worldwide for the Clinical Management of Obesity, 2011-2019 ” by MedMarket Diligence, LLC .
To order the report or ask for sample pages contact [email protected]
Contacts
MarketPublishers, Ltd.
Mrs. Alla Martin
Tel: +44 208 144 6009
Fax: +44 207 900 3970
[email protected]
www.marketpublishers.com
Analytics & News