Legislative Changes to Hamper Innovative Drugs Market in Central Europe, Reveals PMR
25 Apr 2012 • by Natalie Aster

Government reimbursement policy in Slovakia is favouring generics, rather than innovative drugs. This is why a slight reduction in the latter is expected in the foreseeable future, whereas the overall market will grow. There are several reasons for this. First of all, in Slovakia the market share of original drugs by value is relatively high in comparison with those of other countries, and this market subdivision, which is quite saturated, will develop at a slower rate than that of generics. Furthermore, the country’s policy in the pharmaceutical arena does not support innovative companies. In the five reference pricing rounds which have taken place so far, the prices of several hundred medicines have been reduced, and most of these were innovative medicines. The legal changes are also designed to support the generic market rather than that of innovative medicine. For example, in Slovakia a new reference pricing system (in which a drug price will be set at the level of the lowest price in a basket of prices of all of the EU countries) has come into force, with the aim of reducing medicine prices further. In addition, the prescription of APIs, instead of brand names, has been introduced in the country, and this could boost sales of generic medicines. Obligatory generic substitution at pharmacies is also planned to be standard practice in the country.
CAGR for innovative drug markets in the CE countries analysed, 2012-2014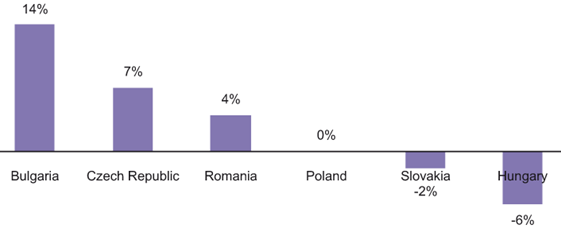
Due to legislative changes implemented during 2011 in most of the Central European (CE) countries, the innovative drug market in the region will decline in 2012 by 1%, to €6.1bn at manufacturer prices, according to the latest report by PMR, entitled “Generic and innovative drugs market in Central Europe 2012”. In all, the CAGR for innovative drugs in the region will be approximately 2% in 2012-2014.
Report Details:
Generic and innovative drugs market in Central Europe 2012
Published: April, 2012
Pages: 303
Price: US$ 3.500,00
In terms of the rate of change on the innovative market, huge differences are to be observed in the development of this subgroup in individual countries. In Bulgaria the growth rate will be substantial – around 14% per annum on average, whereas in the Czech Republic and Romania it will fluctuate around 7% and 4% respectively. In Poland the innovative drug market is expected to develop at a 0% rate and, in 2014, it should, therefore, return to its 2011 value. In Slovakia and Hungary the year-on-year changes will be negative. On the other hand, in all of the CE countries analysed, between 2012 and 2014 the average annual rates of change in the value of the generic drugs market will be positive.
More information can be found in the report “Generic and innovative drugs market in Central Europe 2012” by PMR.
To order the report or ask for sample pages contact [email protected]
Contacts
MarketPublishers, Ltd.
Tanya Rezler
Tel: +357 96 030922
Fax: +44 207 900 3970
[email protected]
MarketPublishers.com
Analytics & News
