Pharma Clinical Trial Services: World Market 2011-2021
19 Oct 2011 • by Natalie Aster

One of the biggest areas of growth in the Japanese clinical trial services market is joint international trials. In order to reduce the drug lag and increase the number of innovative medicines reaching the market in Japan, the Ministry of Health, Labour and Welfare (MHLW) made promoting joint international trials one of the key areas of its five year strategy in April 2007. Also in 2007, the Pharmaceuticals and Medical Devices Agency (PMDA) released guidelines to the industry on conducting joint international trials. Such trials represent a major opportunity for CROs in Japan, particularly those with experience in conducting large multinational trials.
The study “Pharma Clinical Trial Services: World Market 2011-2021” by Visiongain discusses the activities and potentials of Quintiles, Covance, PPD, Charles River Laboratories, Parexel International, ICON, Kendle and other CROs. It provides data, analysis and opinion to benefit your research, calculations, meetings and presentations.
Report Details:
Pharma Clinical Trial Services: World Market 2011-2021
Published: July 2011
Pages: 161
Price: US$ 2,350.00
Report Sample Abstract
The Japanese Market 2011-2021
While one solution for overcoming the drug lag – joint international trials – will drive growth in the clinical trial services market in Japan. The other solution – accepting foreign trial data – will restrain growth. During the forecast period, Visiongain predicts that a greater number of trials will be off-shored to neighbouring countries in Asia. Therefore, despite continuing to grow throughout the period, the Japanese clinical trials services market will lose market share to emerging economies. By 2010, the market will be valued at $3.48bn, up from $1.58bn in 2021. Market share will fall from 7.3% to 5.2% over the same period.
Figure 7.12 Japanese Pharmaceutical Clinical Trial Services: Market Forecast, 2010-2021
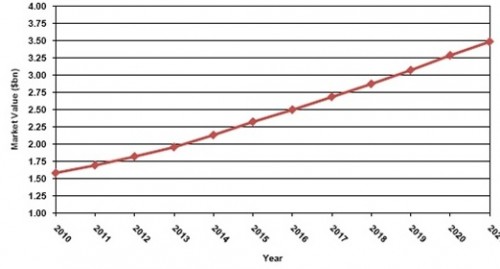
Source: Visiongain 2011
The Brazilian Market 2011-2021
Brazil’s clinical trials market has grown rapidly since the first CROs were founded in the 1980s. However, a national association for these companies did not exist until 2006, when the Associacao Brasileira de CROs (Abracro) was established. In 2010 the Brazilian clinical trial services market was worth $0.28bn, forming only 1.3% of the global market. However, interest in the region has been increasing in recent years, as shown by Chiltern International’s acquisition of local CRO Vigiun in 2009. There are a number of advantages to conducting clinical trials in Brazil, including the following:
- The country has a large, ethnically diverse population. More than 180 million people live in Brazil, many in the large, densely populated cities.
- Many of the clinical trial sites are also located within these large cities. Almost one quarter of clinical trial sites is located in Sao Paulo, for example.
More information can be found in the report “Pharma Clinical Trial Services: World Market 2011-2021” by Visiongain.
To order the report or ask for sample pages contact [email protected]
Contacts
MarketPublishers, Ltd.
Tanya Rezler
Tel: +357 96 030922
Fax: +44 207 900 3970
Analytics & News
