Drug in Focus: Zoledronic Acid
04 Oct 2011 • by Natalie Aster

The following data is extracted from GenericsWeb Pipeline Patent Intelligence and is intended to give a brief outline of factors affecting the potential launch of generic equivalents of Novartis’ blockbuster bisphosphonate Zoledronic Acid. Zoledronic Acid is indicated for the treatment of oncology-related bone conditions (Zometa) and non-oncology bone-related conditions (Reclast/Aclasta) with reported global sales of USD $1.5bn and USD $580m in 2010 respectively.
You can find the available report in the core version (7 country coverage), or in its extended version (44 country coverage). To have the report with full details, please refer to the corresponding Pipeline Developer report.
General information
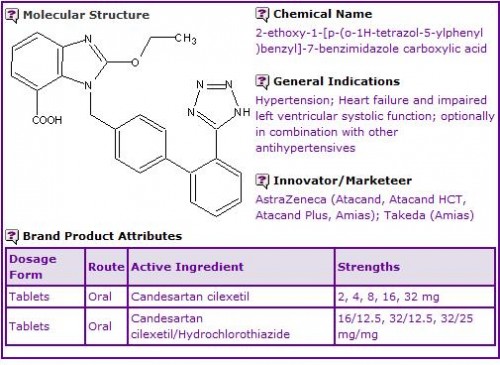
Zometa (4mg/5mL) and Aclasta (5mg/100mL) are currently available for intravenous (IV) administration, the frequency of administration depending on the indicated bone condition under treatment.
Figure 1: General Information table for Zoledronic Acid
INN Constraint Comment
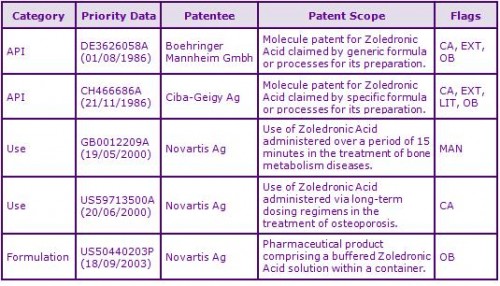
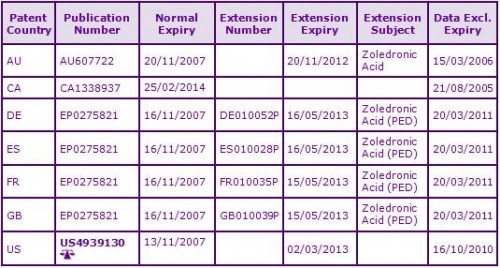
Two patent families to different patentees currently protect the Zoledronic Acid molecule (see Figure 2). Boehringer Mannheim/Roche’s earlier patent family DE3626058A has been extended via Supplementary Protection Certificates (SPCs) in Europe and a national patent term extension in Australia, expiring July 2012. The Canadian equivalent expires in February 2014 due to a ‘17 year from grant’ term calculation. Roche’s patent protection has already expired in the US.
Novartis, the marketing authorisation holder for Zoledronic Acid, is the registered patentee of the second molecule patent family CH466686A. Many SPCs have also been granted based on the European patent in this family, which expire in November 2012. However, Novartis has successfully complied with the measures in agreed paediatric investigation plans (PIP) and has been awarded 6 month extensions to the terms of the SPCs, giving an expiry of May 2013 in most major European territories. National patent term extensions have also been granted in Australia and the US (see Figure 3), the latter also benefitting from a 6 month paediatric extension. Certain patents in this second family claim processes for the preparation of Zoledronic Acid only and, as such, are subject to possible circumvention.
A third patent family GB0012209A, contains patent applications with possible expiries of May 2021 if granted, and claim the use of Zoledronic Acid in the treatment of bone metabolism diseases, where 4mg of Zoledronic Acid is intravenously administered over a period of 15 minutes. If granted with their current claims, such patents may be considered constraining for the 4mg (Zometa) dosage forms.
A fourth patent family US59713500A, with expiries of June 2021, claims the use of Zoledronic Acid in the treatment of osteoporosis; where Zoledronic Acid is intravenously administered intermittently, with periods of 6 months or longer between administrations. Granted patents in this family may be considered constraining for certain indications for Zoledronic Acid products.
A fifth patent family US50440203P claims a ready to use pharmaceutical product comprising a container and a unit dose solution containing Zoledronic Acid, and as such is not considered to be a constraint to generic competition because the protected technology is likely to be circumvented.
Figure 2: Key Patent Indicator; the most significant patents protecting products containing Zoledronic Acid
Figure 3: Patent Family View priority application CH466686A
Figure 4: Marketing Authorisations for products containing containing Zoledronic Acid in Key Countries
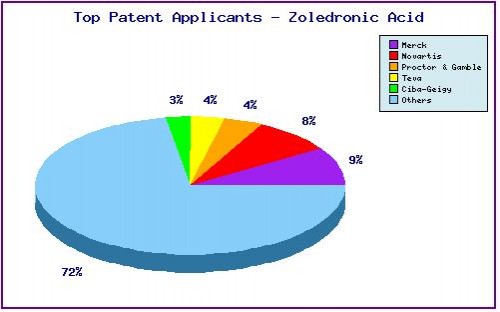
A representation of patentees who have filed the most patent families for this INN. Note the prominence of generic players KRKA and Teva as well as the diversity of applicants represented by the large portion outside the top five. The filings linked to innovators Novartis and Merck generally concern related use and combination patents, whereas KRKA and Teva have focused relatively more on molecular forms and process patents.
Figure 5: Top Patent Applicants
A representation of patentees who have filed the most patent families for this INN. Note the prominence of relevant applications filed by Merck, manufacturer of the competing bisphosphonate Alendronic Acid (Fosamax); and the prominence of generic player Teva as well as the diversity of applicants represented by the significant portion outside the top five.
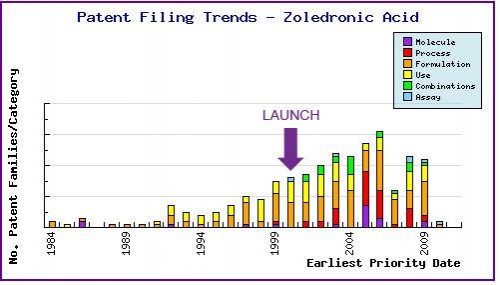
Figure 6: Patent Filing Trend
Represents the timing of the earliest priority filing date for each patent family identified for this molecule as well as the type of claims found in the applications. Note the regular filing of patent applications in the area of formulation, and use patents since the initial approval of Zometa in 2000 and the later approval of Aclasta (2005-2007).
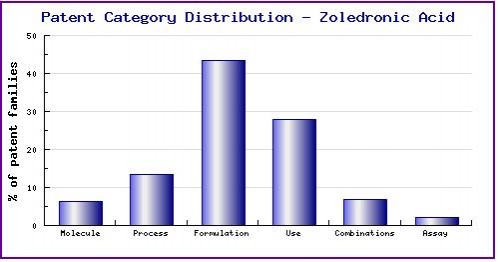
Figure 7: Patent Category Distribution
The types, number and relative distribution of patents that have been filed for Zoledronic Acid obtained via a comprehensive patent search (Pipeline Developer report). Note the dominance of formulation patent families, which alongside oral formulations include several other administration-specific formulations; and the strong presence of use families covering an extensive range of indications, which often differ to the currently approved indications.
In summary, Novartis’ protection remains in force for the Zoledronic Acid molecule in Europe as a result of SPCs and extended patents in other major territories and as such represents a barrier to generic competition. Considering the global sales of Zoledronic Acid the expiry of this molecule protection means that generic competition will undoubtedly be very strong once molecule protection expires, provided later filed patents are not granted in a manner where the claims cannot be circumvented.
To order the report or ask for sample pages contact [email protected]
CONTACTS
The Market Publishers, Ltd.
Tanya Rezler
Tel: +44 208 144 6009
Fax: +44 207 900 3970
[email protected]
www.MarketPublishers.com
Analytics & News