Acute Coronary Syndrome (ACS) Drug Market Prospects 2011-2021
21 Sep 2011 • by Natalie Aster

The study “Acute Coronary Syndrome (ACS) Drug Market Prospects 2011-2021” by Visiongain covers ACS treatments, including those for unstable angina (UA) and acute myocardial infarction (AMI). It also shows commercial prospects for platelet aggregation inhibitors, anticoagulant treatments, fibrinolytic agents and their subgroups to 2021. The report provides revenue forecasts, growth rates, market shares, a SWOT/STEP review, an R&D review. It includes 78 tables and charts and a research interview.
According to industry estimates, the average review time for ANDA submissions is 27 months, which is reduced to about 13 months in case of priority review. Arixtra is receiving priority review since it is the first generic filed for the drug, but has not been approved yet. We expect the generic to launch by 2H 2011. Alchemia also plans to launch the drug in EU once data exclusivity expires in 2012. Another company, Apicore submitted a drug master file (DMF) for fondaparinux sodium in July 2010. However, it has not filed an ANDA yet for its generic drug, giving Alchemia a head start over Apicore.
Report Details:
Acute Coronary Syndrome (ACS) Drug Market Prospects 2011-2021
Published: May 2011
Pages: 132
Price: US$ 2,461.00
Report Sample Abstract
Sales Forecast for Arixtra, 2011-2021
It was earlier expected that the US approval of Arixtra for the ACS indication will accelerate the sales of the drug. However, it now seems that GSK has discontinued the development of Arixtra for ACS in the US. Thus, we only focus on the sales of drug outside the US. Visiongain predicts that there will be an increase in sales of Arixtra from 2010 to 2012, outside the US. The drug stands to lose market exclusivity in the EU in 2012, opening the market to generic competition. Visiongain believes, the generics for Arixtra will already be available in the US market before 2012 and will reach the EU markets as soon as the exclusivity expires. Sales of Arixtra will peak at $151m in 2012, after which time sales will steadily decline throughout the remainder of the forecast period (Table 6.8 and Figure 6.5). Sales will decline to $115m by 2015 (a CAGR of -1.4% from 2010-2015) and $87m by 2021 (a CAGR of -4.5% from 2015-2021). Sales of Arixtra will also be affected by competition from new anticoagulants when these are launched. Arixtra will face competition from Sanofi Aventis’ Otamixaban in particular, we predict.
Table 6.8 Arixtra Sales Forecast ($m), 2010-2021
|
|
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
|
|
124 |
138 |
151 |
136 |
124 |
115 |
107 |
100 |
95 |
91 |
89 |
87 |
|
Annual Growth (%) |
|
11 |
10 |
-10 |
-9 |
-7 |
-7 |
-7 |
-5 |
-4 |
-2 |
-2 |
|
CAGR (%) |
|
|
|
|
|
-1.4 |
|
|
|
|
|
-4.5 |
Source: Visiongain 2011; CAGR values are for 2010-2015 and 2015-2021.
^Sales for indications of Arixtra in the EU
Figure 6.5 Arixtra Sales Forecast ($m), 2010-2021
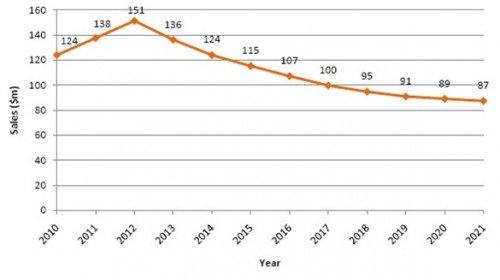
Source: Visiongain 2011
Oral Anticoagulants: Coumadin (Warfarin), Bristol-Myers Squibb
Currently, warfarin (a vitamin K antagonist) is the only oral anticoagulant available for secondary prevention of ACS. The major indications for warfarin are prophylaxis and/or treatment of venous thrombosis and pulmonary embolism. Studies have shown its efficacy in preventing reinfarction and death in infarct survivors. However, owing to high risk of bleeding the drug is only recommended if there is an indication for oral anticoagulation including atrial fibrillation, left ventricular thrombus, and mechanical prosthetic heart valves. Bristol-Myers Squibb's Coumadin (warfarin sodium) is the leading brand available in the market. The drug was launched in 1984 and its generics are widely available in the market.
The high risk of bleeding associated with warfarin intake makes regular monitoring and blood tests to be carried out to monitor the treatment intensity. Several pharmaceutical companies are developing novel oral anticoagulant drugs to replace warfarin.
More information can be found in the report “Acute Coronary Syndrome (ACS) Drug Market Prospects 2011-2021” by Visiongain.
To order the report or ask for sample pages contact [email protected]
Contacts
MarketPublishers, Ltd.
Tanya Rezler
Tel: +357 96 030922
Fax: +44 207 900 3970
Analytics & News
