The Drug-Eluting Stent Market Outlook to 2016
23 May 2011 • by Natalie Aster

The DES market has seen tremendous growth since the introduction of DES in 2003, but the market suffered setbacks in 2007 on the release of clinical data that indicated a link to increased thrombosis. Sales of DES have since stabilized owing to the introduction of novel stents with proven superior therapeutic properties over the traditional bare metal stent.
According to the new report “The Drug-Eluting Stent Market Outlook to 2016” by Business Insights the DES market has been traditionally characterized by low levels of innovation. However, with the shift in hospital purchasing models from the current sole-supplier model to the capitation pricing model, innovative products are the only ones likely to command premium prices. Thus, this will increase the level of innovation in this segment.
The DES markets in India and China are set to undergo growth in the next five years, concurrent with the improvement of the economies of these two countries and the introduction of the ‘Healthy China 2020’ plan. However, leading players face stiff competition from local companies in these regions.
Hospitals are currently entering into preferred-supplier agreements whereby they source their medical device requirements from one or two preferred suppliers to leverage volume-based discounts. This puts large suppliers such as Boston Scientific in an advantageous position as they can cater to consolidated spend categories.
Report Details:
The Drug-Eluting Stent Market Outlook to 2016
Published: March 2011
Pages: 115
Price: US$ 3,835.00
Report Sample Abstract
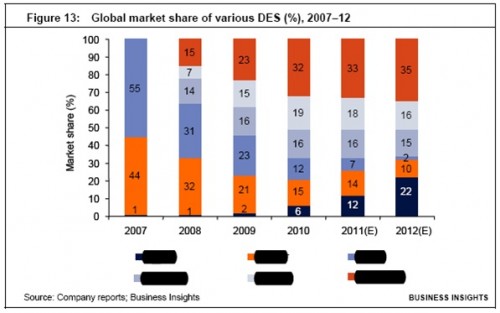
The market share of Promus stents has increased when compared with the Taxus stents, a fact that is attributable to their superior clinical properties. The market share of Endeavor has increased by about 2 percentage points from 2008 to 2010, owing to the expansion strategies of Medtronic in APAC and a strong distribution channel both in the US and in APAC. Cypher has lost significant market share over the past three years, as it has a higher risk of both restenosis and thrombosis when compared with the Promus and XIENCE V labels.
Medtronic: Company overview
Medtronic is a global technology company involved in the manufacturing of medical devices under seven different categories: cardiac rhythm management, spinal, cardiovascular, neuromodulation, surgical technologies, and physio-control. Medtronic operates in more than 120 countries and is headquartered in Minneapolis, US. Regional headquarters are present in Switzerland and Japan. Cardiovascular products are Medtronic’s third largest business division and include angioplasty technologies, stent grafts, heart valves and valve repair technology, and open heart and coronary bypass graft products.
Financial performance
Sales of DES in Medtronic experienced positive growth until 2009. Sales in 2010 fell by about 4.2% as a significant market share was captured by XIENCE V. Sales are likely to increase in future with the introduction of the Resolute Integrity stent in 2011.
More information can be found in the report “The Drug-Eluting Stent Market Outlook to 2016” by Business Insights.
To order the report or ask for sample pages contact [email protected]
CONTACTS
The Market Publishers, Ltd.
Mrs. Alla Martin
Tel: +44 208 144 6009
Fax: +44 207 900 3970
[email protected]
www.marketpublishers.com
Analytics & News
